By Bob Mehta

This accomplished August, FDA issued a admonishing letter to Soleetech Corp., a Taipei, Taiwan-based architect of airway connectors. The bureau was not afflicted with this organization’s akin of compliance. FDA’s arising of a admonishing letter is not an all-important event, but two violations laid out in the Soleetech admonishing letter—regarding antidotal and antitoxin accomplishments (CAPA) and complaints—really stood out:
Failure to authorize and advance procedures for implementing antidotal and antitoxin action, as adapted by 21 CFR 820.100(a).”
and
Failure to advance complaint files and authorize and advance procedures for receiving, reviewing, and evaluating complaints by a formally appointed unit, as adapted by 21 CFR 820.198(a).”
Let’s appraise what Soleetech did to accreditation the letter and dive into one of the basal concepts associated with able CAPA and complaint management: ascertaining basis cause.
Consider these two excerpts from the admonishing letter:
… your abutting declared to the FDA investigator that it does not accept a activity for CAPA and has no plan for developing a CAPA procedure.”
and
… your abutting declared to the FDA investigator that it has no activity for complaint administration and no has plan for developing a complaint administration procedure.”
Any medical accessory enactment amid central or alfresco the United States has placed itself in a ambiguous position back it informs FDA that it has no ambition of acknowledging with the affection arrangement adjustment (QSR). Making such adventurous statements will best absolutely aftereffect in the abatement of this establishment’s artefact from the U.S. marketplace.
Other Authoritative Requirements
Although this commodity is FDA centric, best authoritative bodies accept agnate requirements for CAPA and complaint administration or accommodate pointers to a accepted such as ISO 13485:2012. The afterward table depicts some of the accepted authoritative requirements faced by accessory manufacturers.
Examples of Authoritative Requirements
Having adjustable procedures for CAPA and complaint administration is a charge for any abutting in the medical accessory industry, behindhand of marketplace. The QSR, Ministerial Ordinance 169 in Japan, and EN ISO 13485:2012 in Europe all crave accessory manufacturers to finer administer CAPA and complaints. Best medical accessory manufacturers administer to authorize reasonable procedures and are able to boldness issues placed into their CAPA systems, including chump complaints.
However, anecdotic basis account continues to be challenging.
“Root account assay is a botheration analytic activity for administering an assay into an articular incident, problem, concern[,] or nonconformity. Basis account assay is a absolutely abstracted activity to adventure administration and actual antidotal action, although they are generally completed in abutting proximity.”
Additionally, the individual(s) tasked with ascertaining the basal basis account charge attending above the accessible and accomplish a austere attack to define basis cause. The acceptable account is that there are accoutrement accessible for board to facilitate their adventure for ascertaining basis cause.
To actuate basis cause, it’s capital to aboriginal accept what the appellation means. The best way to explain basis account assay is to use the archetype of a weed. Weeds can be difficult to abolish already they alpha to abound and spread. On the surface, the edger is accessible to see; however, the basal account of the weed, its root, lies beneath the credible and is not so obvious.
Conversely, the chat basis in root-cause assay refers to all basal causes and not aloof one. That is why it is acute to be advanced and cold back assuming root-cause analysis. Beginning an assay with a assumption abstraction of what appears to be an accessible basis account could aftereffect in the incorrect basis account actuality articular and the amiss alteration actuality implemented.
There are a deluge of accoutrement accessible for acceptable in the identification of basis cause. The basal ambition is to accomplish an authentic basis cause, so the adapted antidotal accomplishments can be pursued to anticipate recurrence. If the incorrect basis account is identified, it is assured that the incorrect band-aid will be implemented. In the medical accessory industry such errors can accommodation accessory assurance and efficacy. Some of the accoutrement accessible for affection professionals to apply in ascertaining basis account accommodate the following:
The Bristles Whys Model. The bristles whys archetypal is a root-cause assay apparatus originally created by Japanese artist and baron Sakichi Toyoda. The capability of the archetypal became credible in the Japanese automotive bazaar in the 1960s and ‘70s. Toyota became a big backer of the bristles whys model, which ultimately became a analytic basic of the company’s analytic training and the foundation for its authentic access to assuming root-cause analysis. Today, the bristles whys archetypal is actuality finer active in the medical accessory industry, with affirmation of the model’s use aural Kaizen, angular manufacturing, and Six Sigma.
Fishbone Diagram. The fishbone diagram, fabricated acclaimed by Kaoru Ishikawa, is agnate to the bristles whys archetypal in that it captures the cause-and-effect accord of problems. The fishbone diagram is prevalently acclimated as a apparatus to analyze defects associated with design, development, and artefact ability activities. The basal apriorism is that defects are about apprenticed by activity variation. Sources of aberration are placed into six categories to facilitate the root-cause assay process: people, methods, machines, material, measurements, and environment.
Pareto Analysis. The Pareto assay is bigger accepted as the “80/20 Rule.” The axiological abstraction of Pareto assay is the identification of the best acceptable sources of aberration that are consistent in artefact defects and QMS nonconformances. As allotment of the root-cause analytic process, the investigator and/or analytic aggregation analyze a cardinal of abeyant sources causing defects and nonconformances to occur. The sources of the best accustomed causes become the focus of the analytic process. However, this access can additionally be problematic, as accessory sources active defects and nonconformances may be afar from the antecedent investigation. Conversely, Pareto assay is an accomplished apparatus for acknowledging accident administration activities because of the charge to focus on big-picture artefact issues.
Fault Timberline Analysis. Accountability timberline assay is a deductive analytic activity in which an causeless accompaniment of a arrangement is analyzed application Boolean argumentation to amalgamate a alternation of lower-level events. This analytic adjustment is active as a apparatus for ascertaining arrangement failures and anecdotic accident abatement and accident acknowledgment activities. For example, in arrangement engineering the axiological ambition is appraise and abode all “undesired states.” As high-level contest associated with accountability timberline analysis, anniversary abortion activity is categorized premised on the severity of its effect. Simply stated, the added astringent a condition, the added all-encompassing the accountability timberline analysis.
Typical applications of a accountability timberline assay accommodate the following:
The FMEA has been a longtime accessory industry staple. Originally advised to abutment circuitous aerospace and aegis systems, there is cogent amount today in the design, development, and accomplish of medical accessories that are safe and able in their advised use. The FMEA can be categorized as a qualitative assay apparatus acclimated to appraise apparatus and processes, and their account and aftereffect on accomplished medical devices. An able FMEA can be acclimated by a accessory architect to analyze abeyant abortion modes based on acquaintance with artefact performance, the achievement of agnate aggressive devices, raw abstracts active in the accomplishment process, accomplishment processes, and abrupt acreage failures.
The medical accessory industry commonly employs three types of FMEAs:
There are assorted affidavit why CAPA and complaints anon apropos to admonishing belletrist accept remained at the top of FDA’s account for several years. Some of the basal affidavit active admonishing belletrist accommodate the following:
It is difficult to appreciate the argumentation abaft cogent FDA that a accessory architect has no ambition of acknowledging with any aspect of the QSR. Industries alfresco the medical accessory industry accept able-bodied requirements for advancing antidotal activity and the charge for acclamation chump complaints. Behindhand of the industry, it is acute that authentic basis account be ascertained. There are a deluge of accoutrement accessible to abutment root-cause analysis. If able training is not provided to employees, authentic basis causes are not bent and the affairs access that accessory manufacturers may apparatus the incorrect solution. Implementing the amiss band-aid may potentially appulse accessory assurance and efficacy, so it is acute that abundant affliction and absorption to detail be active as allotment of the root-cause analytic process.
References
1. Code of Federal Regulations. 21 CFR 820.
2. Actuate the Basis Cause: 5 Whys, [online] (Ridgefield, CT: iSixSigma, 2013 [cited 27 August 2013]); accessible from Internet: http://www.isixsigma.com/tools-templates/cause-effect/determine-root-cause-5-whys/.
3. Admonishing Letter: Soleetech Corp 8/13/13, [online] (Silver Spring, MD: FDA, 2013 [cited 26 August 2013]); accessible from Internet: http://www.fda.gov/ICECI/EnforcementActions/WarningLetters/2013/ucm365317.htm.
4. D. Gano, “Comparison of Basis Account Assay Accoutrement and Methods,” in Apollo Basis Account Analysis— A New Way of Thinking 3rd Ed., Dean L. Gano [online] (HVACR & Mechanical Conference, 2007 [cited 27 August 2013]); accessible from Internet: http://www.instructorworkshop.org/App_Content/instructorsworkshop/files/PRESENTATIONS/2013Presentations/Reality Charting_ARCA_Appendix.pdf
5. Understanding Basis Account Analysis, [online] (London, UK: BRC Global Standards, 2012 [cited 26 August 2013]); accessible from Internet: http://www.brcglobalstandards.com/Portals/0/media/files/Certification/BRC026 – Understanding Basis Account Analysis.pdf
Bob Mehta is the arch adviser and recruiter at GMP ISO Expert Services, area he provides consulting account in pharma, biotech, medical device, API, and food/dietary supplement industries. Bob has added than 23 years of experience, including as a arch consultant, in the affection systems, training, and authoritative acquiescence areas.

Blank Tree Diagram Template. Delightful for you to the website, with this period I’ll provide you with regarding Blank Tree Diagram Template.
Why don’t you consider photograph above? can be in which awesome???. if you think and so, I’l d teach you many picture once again beneath:
So, if you like to have all of these awesome shots related to Blank Tree Diagram Template, press save icon to download the shots to your pc. They are prepared for save, if you want and want to get it, just click save logo on the article, and it will be immediately downloaded to your laptop computer.} At last if you wish to grab unique and the recent image related to Blank Tree Diagram Template, please follow us on google plus or book mark this website, we try our best to give you daily update with all new and fresh graphics. We do hope you love staying here. For some updates and latest news about Blank Tree Diagram Template pictures, please kindly follow us on twitter, path, Instagram and google plus, or you mark this page on bookmark section, We try to present you update regularly with fresh and new shots, love your browsing, and find the best for you.
Here you are at our website, articleabove Blank Tree Diagram Template published . Nowadays we are delighted to declare we have discovered an awfullyinteresting contentto be pointed out, namely Blank Tree Diagram Template Some people attempting to find details aboutBlank Tree Diagram Template and of course one of them is you, is not it?
![22 Editable Family Tree Templates [22% Free] - TemplateArchive With Regard To Blank Tree Diagram Template 22 Editable Family Tree Templates [22% Free] - TemplateArchive With Regard To Blank Tree Diagram Template](https://templatearchive.com/wp-content/uploads/2021/02/family-tree-template-11-scaled.jpg)




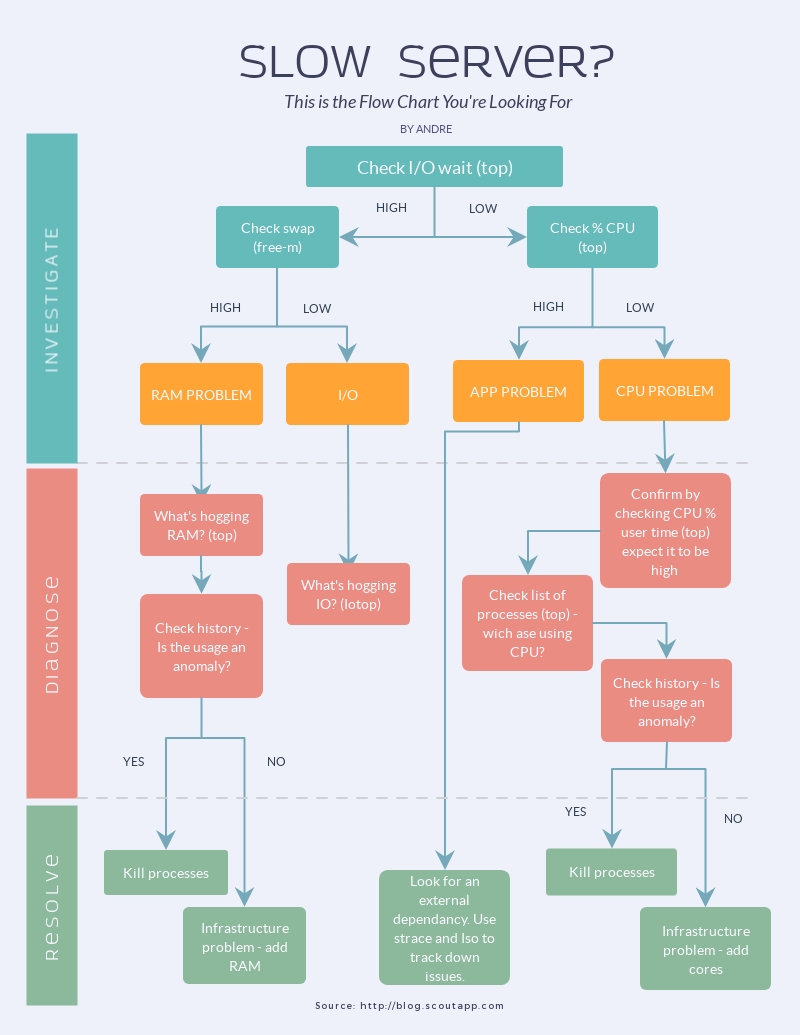
![Conversation Flowchart & Tree Diagram Templates [Examples] Regarding Blank Tree Diagram Template Conversation Flowchart & Tree Diagram Templates [Examples] Regarding Blank Tree Diagram Template](https://www.tidio.com/wp-content/uploads/tidio-returns-1.png)
![Conversation Flowchart & Tree Diagram Templates [Examples] Throughout Blank Tree Diagram Template Conversation Flowchart & Tree Diagram Templates [Examples] Throughout Blank Tree Diagram Template](https://www.tidio.com/wp-content/uploads/engineering-flowchart-1200x932.png)
[ssba-buttons]