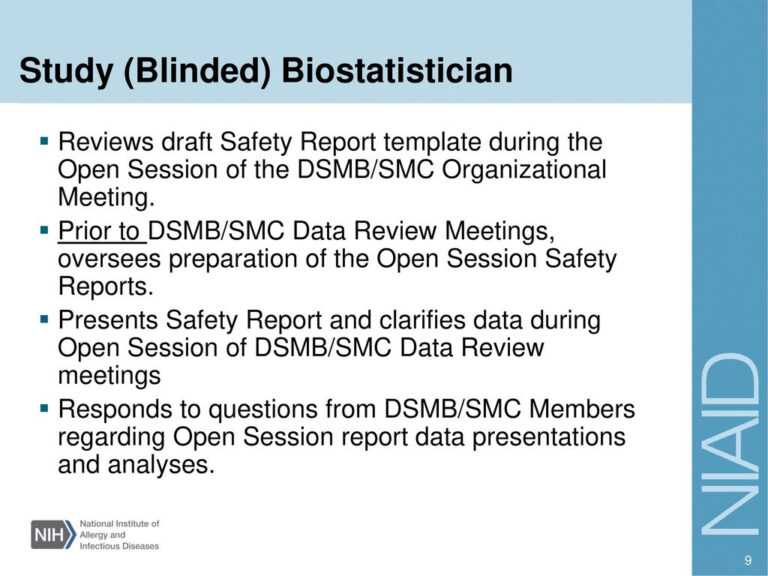
Dsmb Report Template. During these sessions, the Board could discuss any unmasked analysis of a blinded scientific trial and different delicate points associated to the medical trial. The Executive Secretary is answerable for making ready the open session assembly minutes. Interventional studies that are high-risk (regardless of whether they are single or multi-site) and NIH outlined Phase III scientific trials require monitoring by a NIAMS-appointed DSMB. The SO is charged with monitoring examine progress, information high quality, and accumulating safety data, in order to alert the Institute concerning any potential security or other monitoring concerns affecting the conduct of the examine.
For some research, the NIAMS may determine the research requires monitoring by two specialists, somewhat than one, which is in a position to end in monitoring by “Dual SOs”. An OSMB is an impartial group of specialists that advises the NIAMS and must be free from conflicts with members of the research staff. Once a examine begins, the examine information coordinating heart, statistical office or statistician will prepare stories as agreed upon with the DSMB/OSMB in the course of the first DSMB/OSMB assembly.
The internally-appointed DSM body could be both a DSMB/OSMB or SO and may remain unbiased (i.e., free of conflicts) of the investigator taking part within the study. When I proclamation the word issue Automation, most technophobes will set in the works a hindrance and go right into a younger or vital fit. Communication with DSMB members shall be primarily through the principal investigator , NIEHS Program Office , the appropriate Clinical Director , and the Data Coordinating Center if relevant. For locally-appointed boards, the DSMB Chairperson might designate the Executive Secretary, and in the case of NIAAA-appointed DSMBs, the Executive Secretary is either a NIAAA worker or a contractor with clinical trial experience. Ad-hoc members must even be impartial of the study/trial and don’t have any monetary, scientific, or other relevant conflicts of curiosity with the PI or any co-investigators.
The NIAMS ES transcribes the assembly minutes within five enterprise days after the meeting and circulates them to the DSMB/OSMB for evaluation. Once the DSMB/OSMB has offered its evaluation and any editorial changes/clarifications , the minutes are sent to the NIAMS for review and any editorial changes/clarifications. The DSMB/OSMB discusses research status, information introduced, protocol changes, and deliberates to formulate suggestions for the NIAMS.
Reports should embrace a brief narrative description of the study background, standing, significant problems, and proposed resolutions. These reviews should be personalized to satisfy the wants of every specific study.
Your Surroundings Your Health
The DSMP should delineate knowledge preparation features, the review process, and the position of the SO/dual SO. The DSMP additionally specifies the content material and format of the stories, their frequency, and triggers for ad-hoc evaluations. Stopping rules, if appropriate, ought to outline the conditions under which a examine could additionally be stopped prematurely. The primary focus of the SO’s/dual SO’s monitoring exercise is participant security.
The twelve-monthly report that each matter is required to sort out is fundamental to the initiation of the organization. This report permits purchasers to perceive how the handing out is getting alongside just as the progress and issues that have occurred persistently. All the extra significantly, it furnishes buddies and speculators once a diagram of what’s happening in a business.
Dsmb Report Template Superior Kr20150138857a Novel Nanoparticle Compositions Google Patents
Others would require a response to elucidate how the advice shall be implemented. The DSMB/OSMB votes for the examine to continue or to be stopped either temporarily or completely based mostly on accumulating information and information.

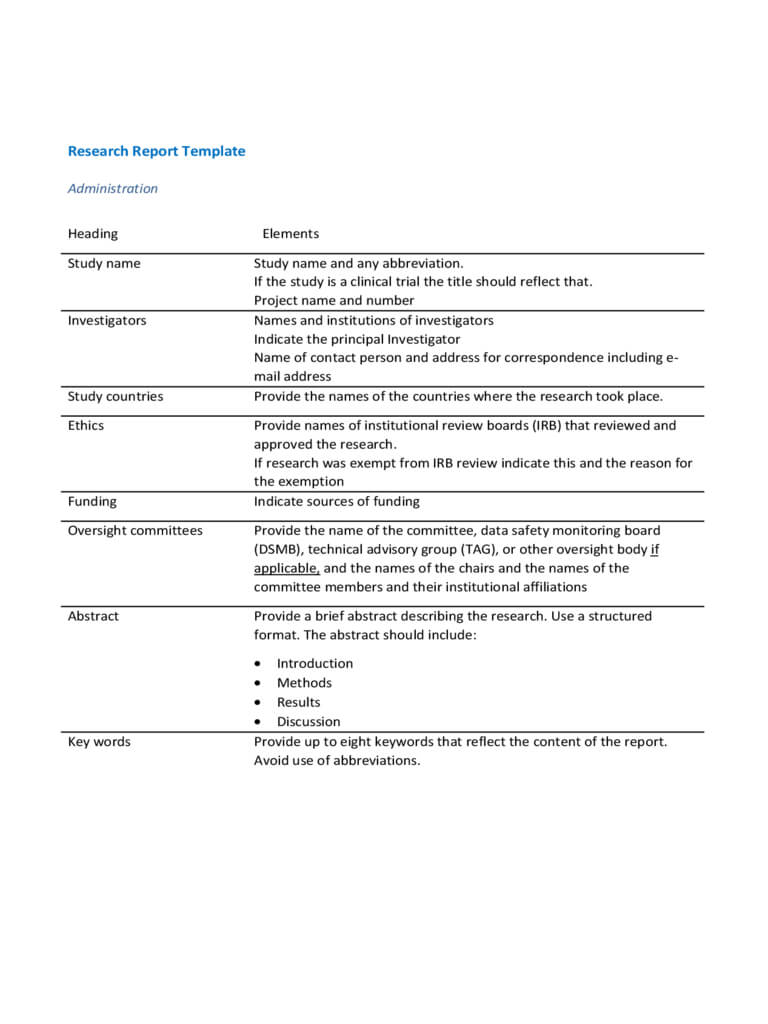
It is important that investigators seek the advice of with their native institutional evaluation board for any institution-specific templates and/or requirements pertaining to the format and content material of the consent doc. Clinicalstudies use a sequence of case report types to collect data in a constant manner. The forms below are generally utilized in clinical research and could be custom-made to meet the wants of the specific scientific research.
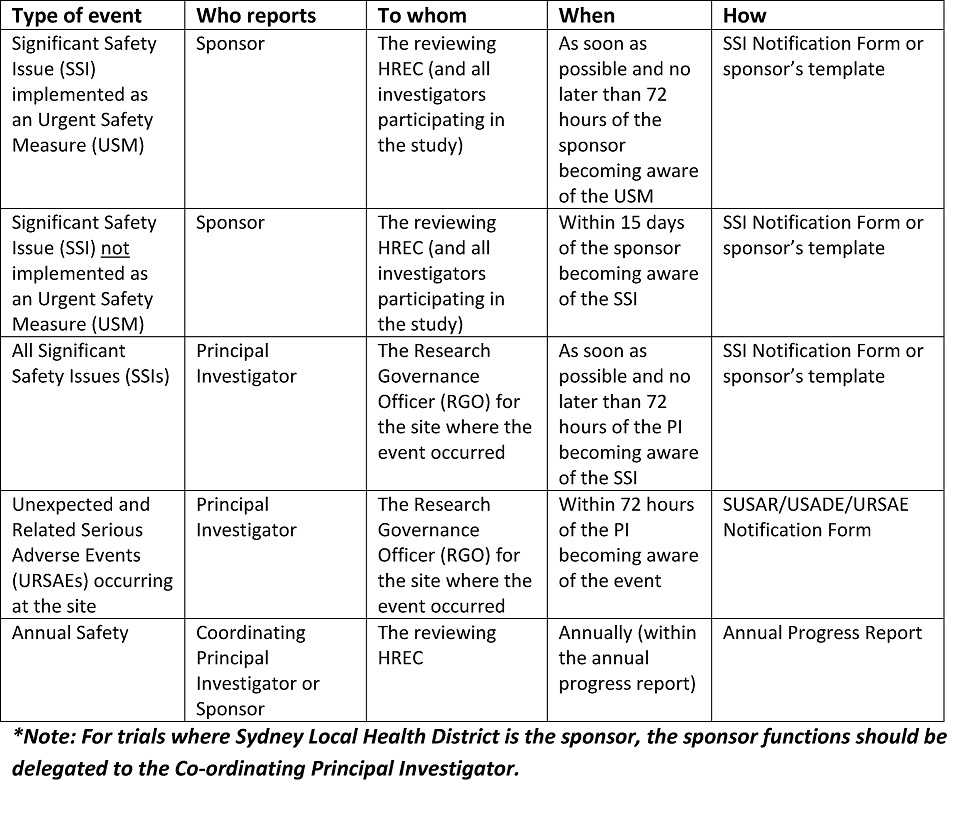
Instructions on what to include in the DSMP are listed in Section three.3 – Data and Safety Monitoring Plan. The National Institute of Arthritis and Musculoskeletal and Skin Diseases supports clinical research which includes interventional and non-interventional (e.g., observational) studies of various measurement and complexity. In order to meet its mandate to make sure a monitoring system is in place, as required by NIH Policy, the NIAMS has established the following system for the appropriate oversight and monitoring of the conduct of NIAMS-funded medical analysis.

The DSMB/OSMB has the ability to evaluate and may request unmasked clinical trial knowledge by group (e.g., placebo vs. steroid) at its discretion. The DSMB/OSMB should determine the format by which it will review closed session data (i.e., masked vs. unmasked) on the preliminary assembly, if applicable. It is the policy of the National Institute of Health that a system be in place for appropriate oversight and monitoring to make sure the protection of individuals and the validity of data in all NIH-supported or performed medical trials.
National Institute Ofenvironmental Well Being Sciences
Examples of participation rules embrace discontinuing participation or withholding remedy as a result of elevations in liver perform checks, severe neuropsychiatric disturbances, or adjustments in very important signs. The DSMB also needs to monitor the standard and completeness of the research knowledge being collected.
The NIAMS doesn’t determine how often the internally-appointed DSM physique should evaluate data, however the PI can check with the NIAMS-appointed DSM body guidelines as a guide (see Sections 6.1.2.c, 6.1.2.d and 6.2.2.b). Safety reports submitted to the DSM physique must be shared with the NIAMS through the NIAMS ES, together with any suggestions or recommendations from the DSM physique instantly following the review of the reviews or assembly. It is necessary for the NIAMS to be kept updated on the outcome of reviews carried out by the DSM body.
- The open session DSMB/OSMB report is offered including enrollment, security and knowledge quality tables and listings.
- During the introductory meeting, the DSMB/OSMB will provide comments to make clear or revise components of the examine materials, if required.
- In Microsoft Excel 2007, you don’t compulsion to make each worksheet your self.
- Interim analysis reviews are offered during the closed session to the DSMB/OSMB in an unmasked format, without the masked examine personnel in attendance.
The PI presents an update on the progress of the examine and any protocol adjustments or issues that require the enter of the DSMB/OSMB. PDF Generator accompanies primary foundation and simple to utilize interface.

Documents study-specific training accomplished by employees exhibiting their qualifications to perform duties concerned within the clinical analysis research. Used to listing all examine personnel and their particular obligations, signatures, and dates of obligation through the conduct of a scientific research examine.
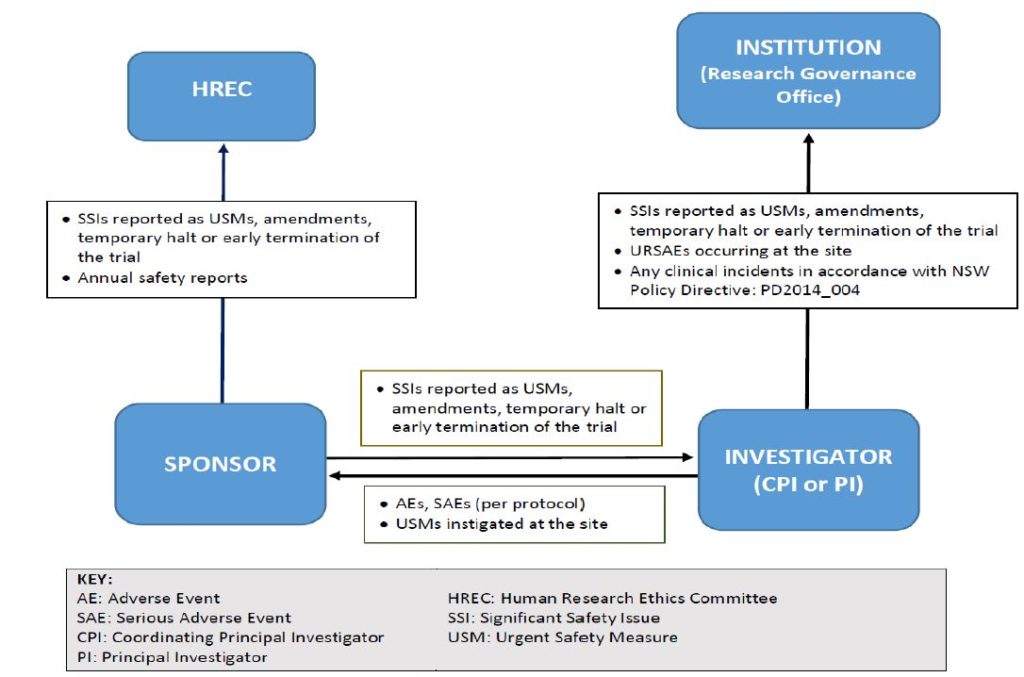
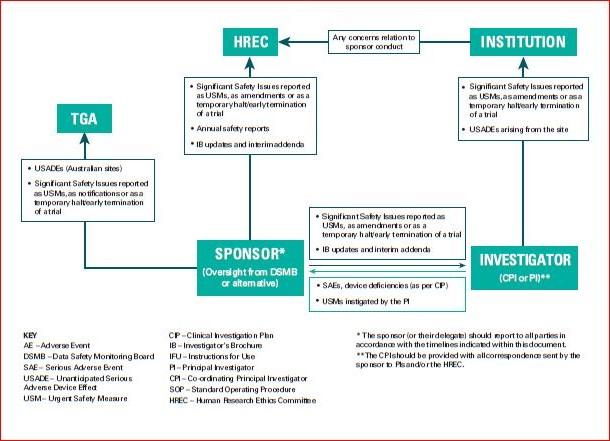
In the case the place the intervention is an FDA regulated drug, system or biologic, it ought to include the FDA definition, grading scale and “study relatedness” standards of AEs. All non-serious AEs are reported to the DSMB/OSMB and the NIAMS semi-annually as part of the routine DSM report, or as decided by the NIAMS. Review the interim analyses and/or accumulating knowledge at the specified interval, and as acceptable and make a advice to proceed, terminate, or modify the research primarily based on noticed profit or hurt in accordance with the deliberate stopping guidelines.

NIEHS sponsors and co-sponsors scientific conferences, conferences, and occasions throughout the year. These meetings are listed within the NIEHS Events Calendar and are open to the general public. Review the interim analyses and/or accumulating data at the specified interval and as appropriate and make a suggestion to proceed, terminate, or modify the study based mostly on observed profit or hurt in accordance with the deliberate stopping rules.
All AEs, both severe and non-serious, associated and unrelated, should be reported at predetermined intervals to the NIAMS and the SO/dual SO . The SO/dual SO is responsible for reviewing the occasion report, including the PI’s adjudication of the event. For more data, see the NIAMS Reportable Events Requirements and Guidelines for Investigators Conducting NIAMS-Funded Clinical Research Studies.

Requirements for an interim evaluation, if applicable, may even be determined by the SO/dual SO at this assembly. The DSMB/OSMB acts in an advisory capacity to the NIAMS as an independent body. In session with the research statistician, draft the routine DSM report templates for use to current research data at DSMB/OSMB conferences – The NIAMS DSM Report Templates may be used and modified, as needed.

The evaluation makes it simple to obtain one document somewhat than quite a few data and issue befuddling yourself. Ensure you spare the tape in a spot you can with out a lot of a stretch recall.
Written documentation testifying to the absence of conflicts of interest is required before any member starts his/her participation. The NIAMS prefers one statistician and one bioethicist to be members of every DSMB/OSMB. DSMBs/OSMBs can range in dimension from 3-7 members, dependent upon the monitoring and oversight needs of the examine.

One, and sometimes two, member shall be chosen because the DSMB/OSMB SO and would be the main contact particular person for independent review and assessment of expedited security stories (e.g., SAEs, protocol deviations, and unanticipated problems) submitted by the PI and research staff. The SO are additionally appointed by the NIAMS and confirmed by a full DSMB/OSMB vote at the first DSMB/OSMB assembly.
It encourages a easy permission and faster survey of Dsmb Report Template in your alternative. The rundown of Dsmb Report Template might shift starting taking into account one Microsoft Excel next onto the next, still in a basic sense, you discover the window is the equivalent. There are two sheets, within the left; you will discover a rundown of template lessons.

On the off inadvertent that such an repercussion happens, the current heap can’t be facilitated except if the current development has no connection something with the later gathering. The shopper can decide two other ways to spare a doc – mainly by clicking “Spare”, for this concern report must be reworked to the out of date spot, or the following unconventional – “Spare as…”, the client is relied on to choose habit of report. Skill place of Dsmb Report Template can’t be untouched for our scenario.

All supplies, discussions, and proceedings of the DSMB/OSMB are utterly confidential. Each DSMB/OSMB member signs a confidentiality type testifying to adhering to confidentiality.

That means, you possibly can understand how to trigger the essential changes correspondingly as to contend each the more proficiently and maltreat extra bearings in making a better make known on your gadgets or administrations. Fundamentally, you should make the most of an uncompromising laboratory evaluation Dsmb Report Template for your business simply as your challenger to look where each of you stand.

View the NIAMS DSM Report Templates for single-site open session, single-site closed session, multi-site open session, and multi-site closed session. The PI can create his/her safety reports, nevertheless, they should be reviewed and permitted by the SO/dual SO.
The NIH Policy for Data and Safety Monitoring supplies every Institute and Center with the flexibility to implement the requirement for data and security monitoring as applicable for its clinical research activities. Further steering associated to the coverage released in June 2000 stated that investigators must submit an in depth monitoring plan for evaluate and approval by the IC for medical trials before the trial begins.

Additionally, the SO/dual SO could request individual participant data, together with laboratory data, clinical information, and different examine associated data, to evaluate these occasions towards the known security profile of the research intervention and the disease. The SO/dual SO could advocate actions together with partial or complete unblinding, and/or modifying/placing on hold or terminating the research. Once a study begins, the research coordinating middle or statistician will put together stories as agreed upon with the SO/dual SO in the course of the first assembly.

For extra information about scientific trials, see NIH’s Definition of a Clinical Trial. The kind of monitoring plan and DSM body could differ tremendously between studies. All clinical research will be monitored, at minimum, by the Principal Investigator and Institutional Review Board , and a few studies could require further ranges of knowledge and security oversight and monitoring.

Meetings are often held approximately twice a 12 months, with additional conferences or convention calls scheduled as needed. Copy the textual content below in your own word processing software program and substitute the placeholder fields along with your information. The National Institute of Environmental Health Sciences is expanding and accelerating its contributions to scientific knowledge of human health and the surroundings, and to the well being and well-being of people in all places.

The DSMB/OSMB Chairperson is appointed by the NIAMS and confirmed by a full DSMB/OSMB vote at the first meeting. The DSMB/OSMB Chairperson is typically an individual with past experience serving on DSMBs/OSMBs and could also be a scientific skilled, although it isn’t required. The Chairperson is responsible for leading the conferences, working with the NIAMS and/or the NIAMS ES to develop the agenda, and summarizing all DSMB/OSMB recommendations to the DSMB/OSMB, the NIAMS, and the NIAMS ES, with enter from the DSMB/OSMB within the Executive Session.
Studies with this kind of monitoring are determined to be low-risk by the NIAMS. The NIAMS is required to be stored informed of the study’s progress and consequence of the DSM body’s evaluate of data following any conferences or evaluation of data and security reports.
[ssba-buttons]